Usage Information
The Electrode
The electrode is an epoxy bodied probe with an electrode at one end. The electrode that makes the electrochemical cell is a silver anode (the long section) and a gold cathode (the end point electrode).
A membrane cap goes over the electrode. When correctly in place, the membrane presses down (lightly) on the gold cathode and electrically isolates it from the silver anode. The membrane cap is filled with an electrolyte solution. The electrolyte solution for this electrode is KCl; it is not advised that NaCl based electrolyte is used as an alternative.

The electrolyte will have absorbed oxygen during storage (more so if the bottle is becoming depleted). This dissolved oxygen in the electrolyte will have to be scavenged out before readings will have any accuracy.
When filling the membrane cap, ensure that no air bubbles are trapped in the cap. If the cap is slightly overfilled to create a positive meniscus, then as you tighten the membrane onto the electrode body, excess electrolyte will escape via the screw thread, therefore take time attaching the cap to let any excess fluid escape.
Preparing the electrode for use.
- Place the membrane cap, with the membrane downward, on a clean surface.
- Half fill the cap with the electrolyte supplied. Tap the cap gently to remove any air bubbles from the electrolyte.
Note: It is important to remove trapped air bubbles because they will feed oxygen to the cathode and hence cause extra signal current to flow regardless of the oxygen concentration in the sample.
- Hold the electrode body vertically (with the cable uppermost) and screw on the membrane cap. One side of the thread is flat which will allow any surplus electrolyte to escape. Unscrew slightly to release any pressure and then gently screw the cap on until it is just tight.
Note: Do not over tighten – the membrane should not be pressurised (bulging away from the cathode tip), as it will become thin and oversensitive.
The assembled electrode must stay connected to an awake sensor unit to keep it polarised. If disconnected, reconnect for 10 minutes before use.
Before use go to Devices and select the appropriate range.
There is a choice of:
- 0 to 150 % sat
- 0 to 250% sat
- 0 to 15 mg L-1
- 0 to 20 mg L-1
Unless the sensor is being used for comparative work it will need calibrating before use.
To calibrate for use in an Aqueous solution e.g. water
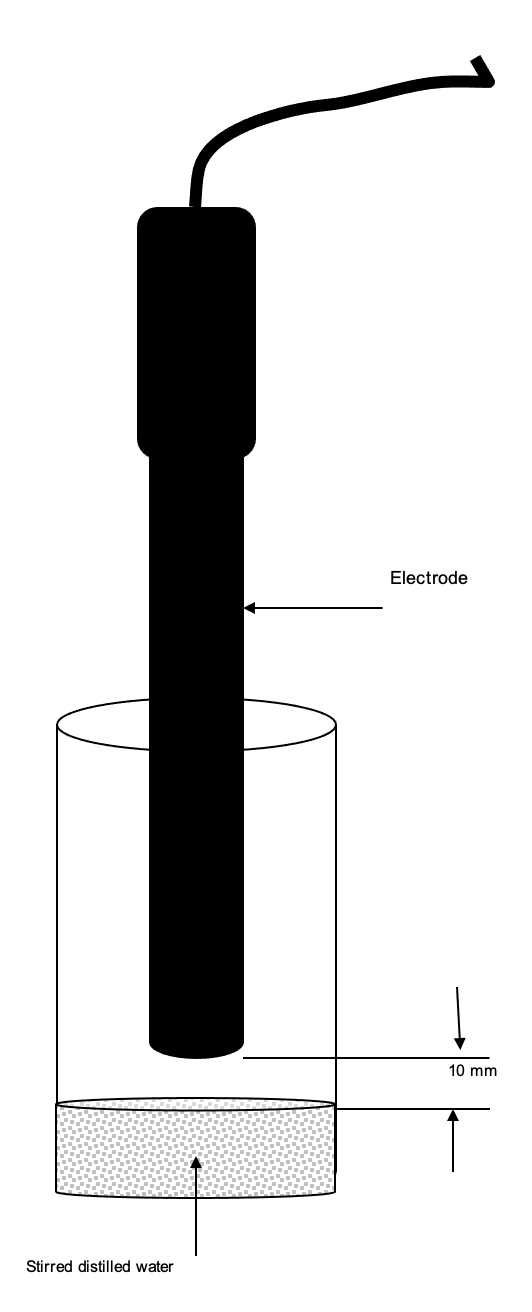
- The range of the Sensor should be set to measure oxygen in water, 0 – 150 (%Sat).
- After the electrode has been polarised for at least 30 minutes, hold the electrode vertically 10 mm above a sample of stirred distilled water (so the air is saturated with water vapour) for about 60 seconds. Do not let the membrane get wet.
- Select Devices > Calibrate
- Follow the instructions.
Using the mg/L range
You will need to supply a value for air pressure and the temperature of the aqueous solution. These functions assume the temperature and pressure are constant and that the effect of dissolved solutes is negligible e.g. water. 1mg/L = 1 ppm.
General Considerations
- The electrochemical reaction within the Sensor removes oxygen from the thin layer of water in contact with the membrane, so it is necessary to renew this layer i.e. by moving the Sensor through the water, or by natural water flow, or by means of a suitable stirrer. This flow must be induced without the entrapment of air bubbles.
- You will notice that as the electrode is positioned deeper in the water that the %dissolved oxygen etc. will change. This is due to pressure in the water column affecting the %oxygen calculation. Therefore make measurements, wherever possible, at the same depth.
- Do not touch the membrane or allow it to touch the bottom of a container.
- Try to keep the electrode vertical during use.
- If the membrane cap is wet, shake gently to remove residual fluid. Do not use a cloth to dry.
- When an electrode is placed in a solution, allow to stabilise before starting to record measurements.
- If you put pH, Oxygen and Conductivity Sensors (electrochemical type) in the same solution at the same time you may notice interference.
- The gas permeable membrane isolates the sensor elements from the solution on test but allows the oxygen to enter. This transport can cause the readings to begin to shift after 4 to 5 hours. If possible, recalibrate every two hours.
- Although the electrode part of the sensor is waterproof, its plug and the Smart Q Oxygen adaptor are not waterproof.
- The Oxygen electrode is supplied with 2 membrane caps, 60 ml of electrolyte and a piece of polishing paper. Further membranes are available as part of the oxygen maintenance kit.
- Treat the membrane carefully as it is fragile. The membrane is PTFE so is very resistant to chemical attack. However it should not be used with wetting agents such as detergents and soaps as they break down surface tension and allow water to pass through the membrane. No grease, oil or organics should come into contact with the membrane.
- Hydrogen Sulphide (H2S) - a by-product of anaerobic respiration recognised by its rotten egg smell - can be a problem as H2S can pass as a gas through the membrane and attack the silver anode to form Ag2S (a black precipitate). The electrode will not function with this coating, which can be difficult to remove. See cleaning.
- The gas permeable membrane may be deformed by pressure if the electrode is immersed to any depth, making the results unreliable.
- The oxygen permeability of the membrane is temperature dependent. This variation in permeability is automatically compensated by the temperature compensation thermistor over the 5 to 45°C operating range. The compensation will lag, the temperature compensator is inside the anode.
- No compensation is provided for the effect of atmospheric pressure. Calibration should therefore be carried out at the pressure at which measurements are to be made.
- Long-term immersion will see drift in the output; the reactions taking place by the silver anode will start to produce less conductive silver salts (mainly AgCl) on the anode and change the conductivity of the anode – cathode circuit. The silver chloride will be seen as dark patches or dulling on the anode.
Cleaning and maintenance
- Take care not to over stretch the membrane when assembling the electrode, as it will become thinner and over sensitive. A membrane that has been damaged in this way will have an opaque white appearance and should be replaced.
- After extensive use, the electrode may become sluggish and erratic due to surface contamination of the gold cathode. If this occurs, polish the tip of the electrode with the fine crocus paper provided i.e. lay the crocus paper on a smooth flat surface, hold the electrode vertically on it and polish gently with a circular motion. Polish only enough to restore to a bright clean surface.
- A net result of the anodes reaction with the electrolyte (KCl) can be a build-up of AgCl on the silver anode. If the anode is covered in an AgCl deposit (black/brown stains) the electrode will give unreliable results. This deposit may be removed by:
- Make up a paste of baking soda (bicarbonate of soda - NaHCO3) with some water, leave the tip in the paste for at least an hour then rub with some of the paste until the tip is clean and shiny. Or
- Soaking the electrode tip overnight in a neat ammonia solution.
- If neither of these methods works use either the crocus paper supplied or jeweller’s rouge to remove the stains. Do not scratch the silver; the surface must remain even, or you will create long-term stability problems.
Note: Ammonia solution is toxic, corrosive and irritant. Please refer to local safety regulations for handling instructions.
